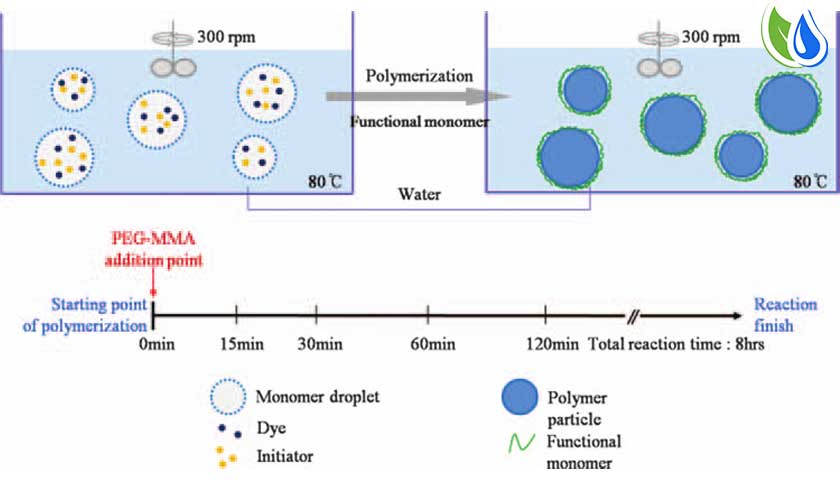
Suspension polymerization
uspension polymerization, sometimes called bead, pearl or granular polymerization, is one of the most widely used polymerization techniques. It is essentially a water or solvent cooled bulk polymerization, though water/solvent soluble initiators may be present that could alter the reaction kinetic.
The suspension process is practiced by only a few companies because it requires a higher degree of production control and product engineering during the polymerization step. This process suspends the water-based reactant in a hydrocarbon-based solvent. The net result is that the suspension polymerization creates the primary polymer particle in the reactor rather than mechanically in post-reaction stages. Performance enhancements can also be made during, or just after, the reaction stage.
Suspension polymerization
Suspension polymerization is a heterogeneous radical polymerization process that uses mechanical agitation to mix a monomer or mixture of monomers in a liquid phase, such as water, while the monomers polymerize, forming spheres of polymer.
This process is used in the production of many commercial resins, including polyvinyl chloride (PVC), a widely used plastic, styrene resins including polystyrene, expanded polystyrene, and high-impact polystyrene, as well as poly(styrene-acrylonitrile) and poly(methyl methacrylate).
Suspension polymerization is a heterogeneous radical polymerization process that uses mechanical agitation to mix a monomer or mixture of monomers in a liquid phase, such as water, while the monomers polymerize, forming spheres of polymer.
This process is used in the production of many commercial resins, including polyvinyl chloride (PVC), a widely used plastic, styrene resins including polystyrene, expanded polystyrene, and high-impact polystyrene, as well as poly(styrene-acrylonitrile) and poly(methyl methacrylate).
IUPAC definition
Polymerization in which polymer is formed in monomer, or monomer-solvent droplets
in a continuous phase that is a nonsolvent for both the monomer and the formed polymer.
Note 1: In suspension polymerization, the initiator is located mainly in the monomer phase.Note 2: Monomer or monomer-solvent droplets in suspension polymerization have
diameters usually exceeding 10 μm.